Everything in the Universe is made from one type of thread.
All workings of the Universe are result from said thread.
The particle itself would be just the grey threads (or strings) in the picture (no color and a lot thinner of course).
It would fit perfectly inside of a
dodecahedron.
Actual thread (or string) length is about one Ångström and it is fine enough where 10 threads (20 radii) could curl-up into the size of a neutron.
ELECTRONS ARE FIXED POSITION
~~~~~~~~~~~~~~~
Bond Angles Are Exact
~~~~~~~~~~~~~~~
Electrons must be fixed position - anything else is impossible.
Notice the way a lot of molecules tend to form tetrahedrally?
A tetrahedron is the opposite diagonal corners of a cube.
And the reason being: electrons form a thread-mesh-type cage around the nucleus. Certain sizes are of course the 8 corners of a cube - that's what the octet rule is and how it happens.
A lot of atoms and molecules have a bond angle of exactly 109.4712º (degrees). That's tetrahedral. - corners of the cube.
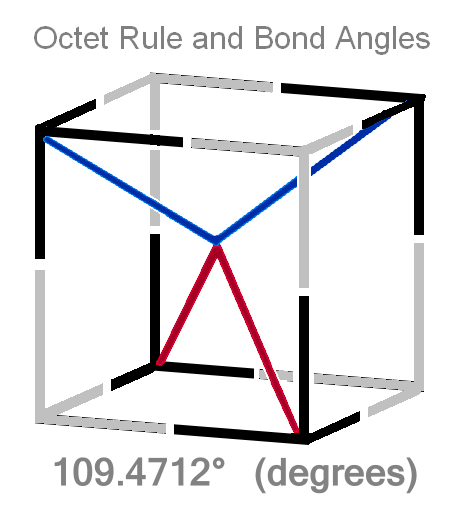
That is NOT probability.
It is NOT a cloud.
It is NOT a blur.
It is NOT uncertainty.
It is NOT counterintuitive.
IT IS EXACT.
Electrons DO NOT have any probability nor uncertainty involved
Electrons actually are something but everyone mistakenly thinks the vibration traveling around the thread is the electron - that's what has caused all the confusion.
The electron is conveying vibrations but the material it is made from (quantum threads) are NOT moving..
EXAMPLE: Think guitar string - the string itself would be the electron but everyone thinks the vibration or note is the electron.
That is why there is all kinds of probability and uncertainty - the vibration is traveling around a spherical thread mesh cage - where exactly is the vibration? No way to know for sure.
Got that? The electron has exact position. The vibration position is of course unknown.
SPECIAL NOTE: Heisenberg would be OK with this. A vibration of course has uncertain position.
~~~~~~~~~~
The Octet Rule
~~~~~~~~~~
The reason why there are bonds, bond order and bond angles is because electrons form a thread mesh-type cage around the nucleus - electrons are fixed positions.
The "Octet Rule" is a perfect 8 corner cubic structure of electrons.
It not only explains everything - it makes the explanations an easy feat.
EXAMPLE: They don't know how methane CH
4 can form?
It's an easy answer...
Methane (one carbon atom and 4 hydrogen atoms) CH
4 is the greatest example of fixed position electrons.
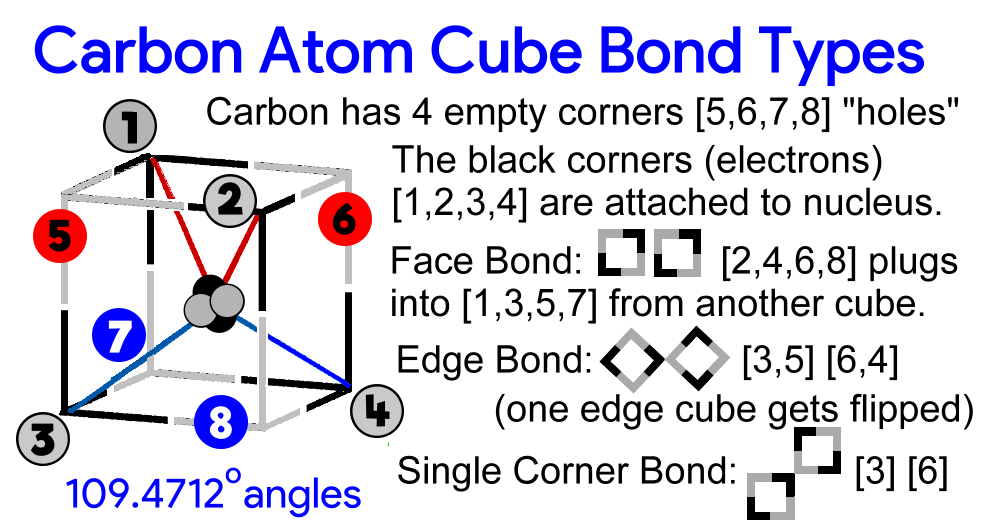
There would be a cubic carbon atom and 4 corners would each have an hydrogen atom electron fixed into place.
If the picture is thought of as a carbon atom - four electrons are fixed to the nucleus. The other four (grey) empty spots can accept electrons from other atoms - in this case hydrogen.
 ~~~~~~~~~~~
~~~~~~~~~~~
Sigma Pi Bonds
~~~~~~~~~~~
Notice in the picture every side or face of the carbon atom has 2 electrons (the black) attached to the nucleus and 2 electron empty spots (the grey) ready to be filled in.
Pi Bond
If you stack cubic carbon atoms one on top of the other there is a perfect arrangement of electrons and empty spots. They will fit perfectly together and bond.
Notice: if you start at one nucleus then travel to any edge or corner electrons and keep going in the same direction - it will take you off into space - NOT into the other atom. You need a big angle to get from one nucleus to the other through bonds. That must be why Pi Bonds are supposedly NOT as strong as Sigma Bonds.
Textbook Pi bond (π bond)

Two p-orbitals forming a (π bond).
"In chemistry, pi bonds (π bond) are covalent chemical bonds where two lobes of an orbital on one atom overlap two lobes of an orbital on another atom. Each of these atomic orbitals is zero at a shared nodal plane, passing through the two bonded nuclei. The same plane is also a nodal plane for the molecular orbital of the pi bond." --
Wiki Pi
Notice this explanation of Pi Bonds from Wiki and also the picture with Red Balloons. They know there are four things bonding, they have some sort of plane between them and wiki also shows the bond as some sort of bent ellipsoid - like the
"Cloud Gate."
Quantum Thread Theory Pi Bond (π bond)
Something similar is happening where the Quantum Thread Theory cube faces touch - that is a plane. And the bond angles are also bent and facing each other.
So their explanation is basically correct.
But if you understand Quantum Thread Theory and realize everything is made from threads and electrons are fixed positions you will know the underlying reason for everything.
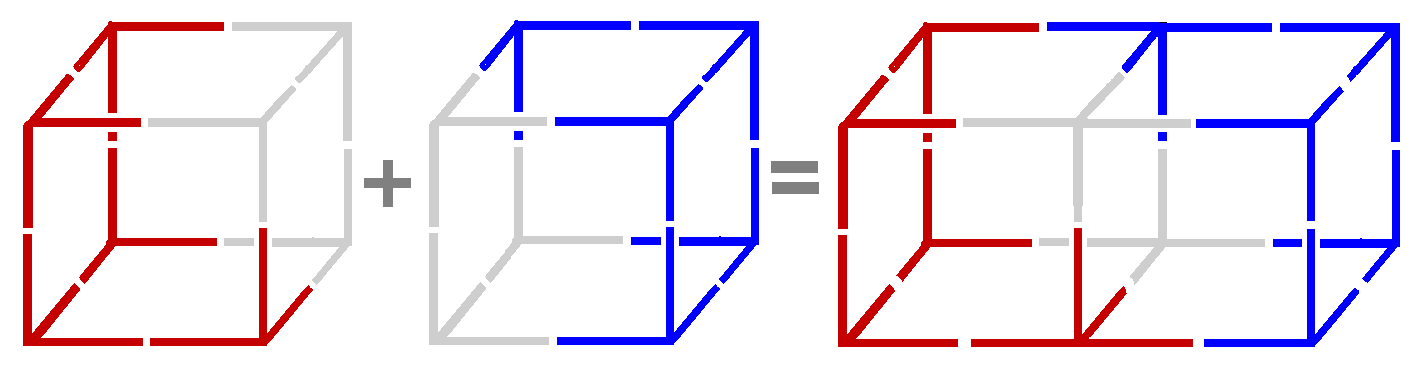
Quantum Thread Theory Sigma Bond (σ bond)

The carbon atoms can also bond on their edges. (a cube has 6 sides (or faces), 8 corners and 12 edges). An edge would have two electrons occupying adjacent corners. Notice in the QTT Sigma bond the adjacent corners that have bonding electrons are on a directional plane that points directly through the other atom nucleus? That's what they are calling "Head-On." From one nucleus to the edge then to the other nucleus is a straight line. That is NOT happening in the the Pi bond.
They call that type of bond a Sigma bond
"In chemistry, sigma bonds (σ bond) are the strongest type of covalent chemical bond.[1] They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most simply defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a (σ bond) is symmetrical with respect to rotation about the bond axis." --
Wiki Sigma
 ~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Nitrogen Gas And The Triple ≡ Bond Trilemma
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~

I did some digging and I found other examples of the "Cubic Atom" from long ago. Everything worked fine and easily explained everything except the triple bond - like those that form from nitrogen gas. So, they disqualified the whole model. That's a shame because the thing that was wrong with the model was the electron. They thought the electron was a tiny point that was fixed (or revolving slightly) at the corners of the cube.
"Landé studied atomic structure intensively for the next seven years. In 1916 Sommerfeld had begun to apply the new atomic theory to form a general quantization rule. Landé's work over cubic and tetrahedral electron trajectories ("cube atoms") became of great interest to Sommerfeld, Peter Debye and Bohr." -- wikipedia
 "Gilbert N. Lewis (dot :: structure) proposed the cubic octet in 1902 as a chemistry teaching tool to give a structural "explanation" for the periodicity of the elements in terms of the electron, which physicist J.J. Thomson had discovered only seven years previously.
"Gilbert N. Lewis (dot :: structure) proposed the cubic octet in 1902 as a chemistry teaching tool to give a structural "explanation" for the periodicity of the elements in terms of the electron, which physicist J.J. Thomson had discovered only seven years previously.
The model was consistent with formation of the Cl-Cl single bond to complete the octet of two chlorine atoms by sharing two electrons along an edge. It was also consistent with formation of the O=O double bond by sharing four electrons across a face. But there was no way it was consistent with formation of a triple bond in N2." -- J.M.McBride
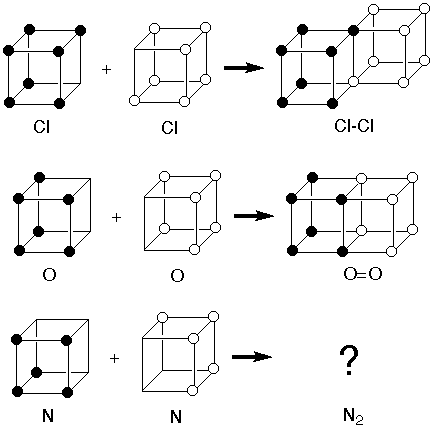 ~~~~~~~~~~
~~~~~~~~~~
All Bond Types
~~~~~~~~~~
 ~~~~~~~~~~~~~~~~~~~~~~~~~~
~~~~~~~~~~~~~~~~~~~~~~~~~~
Quantum Thread Theory Triple ≡ Bond
~~~~~~~~~~~~~~~~~~~~~~~~~~
They also had a problem with fixed position electrons and the energy involved
" There is an even more fundamental objection to the very idea of any static structure for the atom.
In 1839 Samuel Earnshaw had proven that no system governed by inverse square force laws (i.e. where energy is proportional to 1/r and force, the derivative of energy to 1/r2) can possess the local energy minimum that would be necessary if a particle is to have a stable location. Many important forces are of the 1/r2 form. These include gravity, the interaction between charges, and the interaction between magnetic poles." -- J.M.McBride
Quantum Thread Theory eliminates all problems. The electron is fixed position but the threads allow vibrations to traverse the whole atom. That's what the energy is - the vibrations - NOT the threads themselves. Also, the electrons - being made from threads - can connect.
 ~~~~~~~~~~~~~~~~~~~~~~~~~~
~~~~~~~~~~~~~~~~~~~~~~~~~~
The Molecular Structure Of Graphene
~~~~~~~~~~~~~~~~~~~~~~~~~~
All you have to do to make graphene is take a bunch of Pi bonded atoms (that's a group of two) and stack them in a line. Now get some more Pi bonds and flip them over and Sigma bond them to the other Pi bonds.
It's NOT as confusing as it sounds - it's actually very simple.
Here is a picture of what a sample of graphene would look like...

Notice every atom (that's one cubic shape) is bonded to 3 other atoms. All of the atoms now have 8 electrons (the octet rule) and they are also sharing four bonds.

The pattern looked too tall to be the familiar hexagon lattice - that's because the Pi bonds are double so they squeeze together tighter. And since every atom has a PI bond: it distorts the whole lattice.
I initially just decreased the height of the image to try and make it more like the hexagon shape lattice but even though I made a large decrease something was NOT correct about it. So I decreased the height and increased the width - that's what would happen with an actual lattice - if you squeeze the up and down axis it will increase the left and right.
I only had to make a slight change and it just about matches perfectly.

 ~~~~~~~~~~~~~~~~~~~
~~~~~~~~~~~~~~~~~~~
A Solid Sheet Of Graphene?
~~~~~~~~~~~~~~~~~~~

Can carbon atoms form into a solid sheet?
No, if there were a solid layer or sheet of carbon atoms - the middle atom (center cube in picture) is an example of the number of connections and bonds all atoms would have and it is too many. I count 16 connections going to the center cube in the picture. The most a carbon atom can have is 8. That's 4 from the nucleus and 4 from other atoms. An atom could have all 8 connections coming from the nucleus but that would make it Neon - a Noble Gas. Neon already has all corners filled and attached to the same nucleus.
Look at the center cube. It is carbon and it has 16 connections - that's impossible.
Any type of "L" shaped bond is also impossible because of too many corner connections or it leaves empty spots.
~~~~~~~~~~~~~~~~~~~
The Thread Mesh-Type Cage
~~~~~~~~~~~~~~~~~~~
Larger sized elements are unstable because electrons form a thread-mesh-type cage of concentric spheres around the nucleus.
The basic "electron" is made from 10 threads joined at their centers (that forms 20 radii emanating from a common center).
The threads are approx. one angstrom in length.
One thread is attached to the proton, 18 form the electron "disc" and the other thread connects to outer electrons. That's why the periodic table has 18 columns - when electrons join each other they have 18 threads to play with. Got that?
Here are two electrons...
~~~~~∗~~~~~ ~~~~~∗~~~~~
When the atom is small (inner shells) threads can completely twist and connect together.
~~~~~∗≈≈≈≈≈∗~~~~~
But as it gets larger (outer shells) the electrons might be further apart and therefore less thread connection twist
~~~~~∗~~≈≈≈~~∗~~~~~
until the point of instability...
~~~~~∗~~~~≈~~~~∗~~~~~
~~~~~~~~~~~~~~~~~~~~
Every Corner Is An Electron
~~~~~~~~~~~~~~~~~~~~
NOTE: An electron thread goes from the nucleus to the electron disc. There the disc forms one corner of the cube. An electron disc has 18 threads, but they do not reach diagonally across the cube, so the 18 threads bunch together into three groups of 6 threads. All corners actually have 18 threads divided equally and attaching to other corners.
A cube is an automatic shape that forms in the "shell" because the electron threads attach to other electrons based on the amount of electrons and the distance from nucleus - they shoot for a tight around-the-sphere pack.
EXAMPLE: If you opened-up 8 umbrellas; they would form an umbrella ball - something like the spherical cube pack. But if you increased the length of the handles you would need a lot more umbrellas to make and fill-in an umbrella sphere - that would be like an outer shell of an atom.
Any other configuration you can find or think of also proves the electrons are fixed position. It depends on the number of electrons, what shell they are in, if there are double bonds, etc.
QUESTION: If you have an atom or molecule and everything is bonding trigonally at exactly 120° (degrees) ...would you say the electrons are orbiting randomly or do they have a definite fixed position?

Face-centered cubic, body-centered cubic, all crystalline structures. Nothing is random - everything is exact.
So, Yes - electrons are fixed position. And now you know how they work.
Here is a regular thread tension formula...
Tension = velocity squared x mass / Length.
If we plug c in and rearrange we get the one-inch formula... TL = mc^2
http://www.mccelt.com/the-one-inch-equation-to-explain-all-physical-laws.php
Add a Comment



 I did some digging and I found other examples of the "Cubic Atom" from long ago. Everything worked fine and easily explained everything except the triple bond - like those that form from nitrogen gas. So, they disqualified the whole model. That's a shame because the thing that was wrong with the model was the electron. They thought the electron was a tiny point that was fixed (or revolving slightly) at the corners of the cube.
I did some digging and I found other examples of the "Cubic Atom" from long ago. Everything worked fine and easily explained everything except the triple bond - like those that form from nitrogen gas. So, they disqualified the whole model. That's a shame because the thing that was wrong with the model was the electron. They thought the electron was a tiny point that was fixed (or revolving slightly) at the corners of the cube.







 Can carbon atoms form into a solid sheet?
Can carbon atoms form into a solid sheet?

